AI Solutions

Simplifying and Scaling AI Translation Technologies
How AI-driven consolidation will catapult your global content initiatives.


Case Study: Multilingual Retail Marketing
New AI Content Creation Solutions for a Sports and Apparel Giant

- RESOURCES


Global procurement and clinical outsourcing professionals face increasing pressure to implement AI solutions that drive transformative cost-savings, operational efficiencies, and improved oversight of clinical trial costs. Clinical research trials are notoriously expensive. Average pre-launch R&D costs to support a new drug range from $161 million to $4.54 billion (depending on the therapeutic area). The total language services market within the clinical life sciences space was estimated at $1.01 billion as of 2019. The total global addressable market for language services and technology in 2023 was estimated at $2.68 billion.
Once a drug candidate successfully passes phase 1 human trials, clinical development becomes multinational, necessitating extensive language services and localization to support global clinical development plans. However, sponsors are often risk-averse regarding AI for clinical trials and research language outcomes — even though AI is already revolutionizing trials’:
- Design
- Conduct
- Analysis
Lionbridge firmly believes the EU reforms on clinical trial applications are a prime case for AI in life sciences, particularly to support language outcomes and for strategic procurement partnerships with life sciences translation services.
Centralized Submission Dossiers Across the EU: A New Opportunity
Implementing the Clinical Trials Information System (CTIS) in the EU allows clinical trial sponsors and medical device manufacturers to submit a single clinical trial application dossier for multinational studies conducted in the EU. The EU Regulations (CTR, MDR, and IVDR) and the CTIS platform facilitate a joint authorization process across all EU Member States.
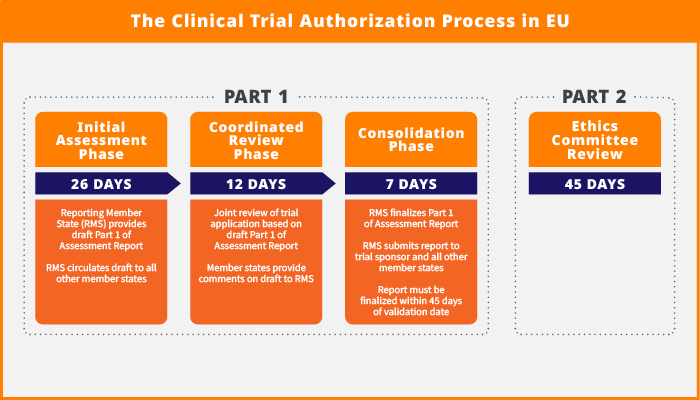
The clinical trial authorization process is streamlined into a 45-day procedure, with one Member State acting as the Reporting Member State (RMS) and responsible for coordinated assessment across all concerned Member States. This process cuts the procedure by 15 days, compared to the previous 60-day process. It includes these sequential steps:
- 26-day assessment phase
- 12-day coordinated review phase
- 7-day consolidation phase
In parallel, during the same 45-day assessment window, Ethics Committees in each Member State review Part 2 of the application dossier.
Language outcomes during the coordinated review and consolidation phases may require rapid revision, or regulators may request additional information in local languages. The trial team must respond quickly to regulatory Request for Information and swiftly manage language activities across all countries to avoid delays or automatic application lapses.
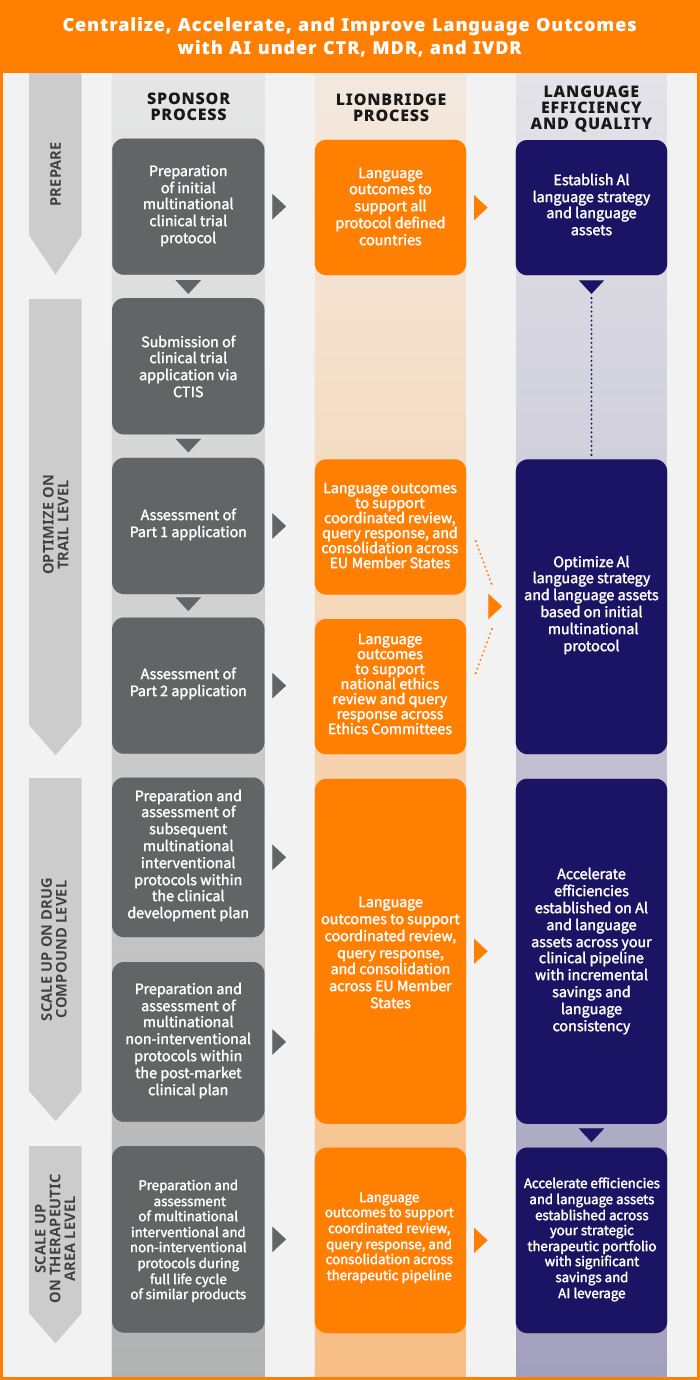
Centralizing Language Services: A Strategic Imperative for AI for Clinical Trials
The strict timelines and tacit decision principles of the revised EU Regulations should encourage sponsors to centralize clinical trial translation services. Managing language outcomes through decentralized local trial management functions contradicts regulators' efforts to harmonize and streamline clinical trial authorization procedures. With many pivotal studies executed across multiple EU countries, de-centralization may become a risk factor in obtaining critical phase 3 milestones. Additionally, by sticking to a multivendor procurement strategy, trial sponsors may miss significant cost-savings derived from using a single AI system and AI translation in clinical trials, including:
- Centralized scaling of translation
- Summation
- Content creation
Multivendor procurement strategies, often dictated by Quality Management Systems, help to ensure business risk mitigation and obtain competitive pricing. However, procuring language outcomes from different language service providers within the same clinical development program is ill-advised and arguably riskier than a centralized approach. Following multivendor QMS procedures, often drives inconsistencies and burdens clinical trial budgets. This is especially cumbersome in an industry that heavily scrutinizes R&D costs.
Trial sponsors can use AI for clinical trials to achieve incremental cost savings, while improving language quality and consistency. This goal can be implemented at any of these levels:
- Trial
- Compound
- Therapeutic
Centralizing Language Outcomes for Combined Drug-Device Studies
AI-supported language outcomes don’t just have potential at the product level. Clinical trials increasingly combine investigation of a medicinal product with the performance study of an In Vitro Diagnostic device or a clinical investigation of a medical device.
Although a lack of alignment has been identified in the clinical trial authorization process across CTR, MDR, and IVDR, a centralized approach to language outcomes can also drive efficiencies for combination studies. Lionbridge recommends using AI for clinical trials’ language strategy across content types for combination studies. Terminologies, language assets, and content can be shared across an In Vitro Diagnostic device combined with medicine to support personalized medicine development. During development of the drug and its companion diagnostic, AI workflows can be predefined and implemented alongside glossaries, helping:
- Drive efficiencies
- Change procedures
- Aligning messaging and performance claims to support mutual endpoints
Treat Your Language Service Provider Like a CRO
With escalating clinical research costs, Contract Research Organizations (CROs) have become essential to successful and efficient clinical trial execution. Over time, their role has evolved from a service provider assuming administrative trial duties to becoming true partners who influence:
- Trial design
- Data management
- Patient recruitment
Digital trial execution and AI for clinical trials have also opened new avenues for strategic partnerships across the B2B relation.
With Large Language Models (LLMs) transforming the entire content journey, sponsors can now engage in similar strategic partnerships with Language Service Providers (LSPs) and fully benefit from AI for clinical trials.
Get in touch.
Ready to explore language and content solutions to support your full drug and medical device development life cycle? Lionbridge offers unique multidisciplinary expertise with LLMs, language excellence, regulations, and life sciences content. We offer advanced and bespoke AI solutions, from planning to creation, analysis, summation, and localization. Let’s get in touch.


